Cell Culture and Processing Facility Attached to the Clinic
5Star Medical Club has a GMP-compliant cell processing facility within our group clinic, Biostyle Clinic. A cell culture and processing facility is a dedicated cleanroom where the necessary level of cleanliness is maintained for culturing cells. Operation of such a facility requires a facility number issued by the Minister of Health, Labour and Welfare, in accordance with the “Act on the Safety of Regenerative Medicine.”
Biostyle Clinic’s Cell Processing Center (CPC) has been registered with the Minister of Health, Labour and Welfare as a facility for manufacturing specified cell products. It cleared Clean Level Class 100 and meets standards equivalent to ISO 5. Compared to other CPCs in Japan, our facility satisfies even stricter, higher-tier standards, ensuring a superior level of safety. Clean Level Class 100 means that there are fewer than 100 particles of 0.5 μm or larger in one cubic foot of air. This level of purity is comparable to the cleanliness required for semiconductor manufacturing plants.

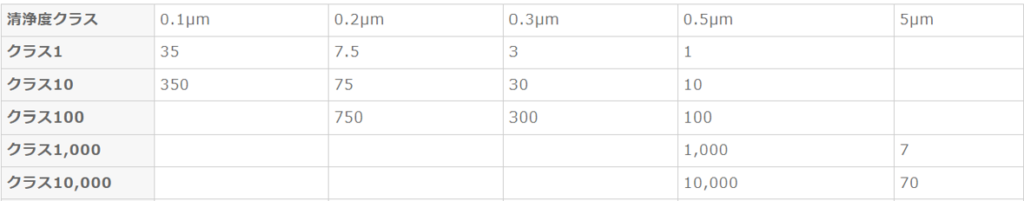
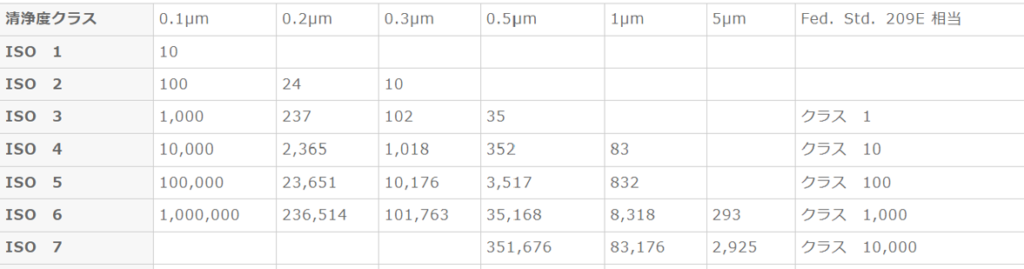
Cleanroom Cleanliness: A cleanroom is a space where airborne microparticles and microorganisms are managed below a specified cleanliness level. Environmental conditions such as temperature, humidity, and pressure are also controlled as needed. Cleanliness (Class) is broadly divided into two standards: [U.S. Federal Standard Fed. Std. 209E] and [JIS B9920 / ISO 14644-1].
U.S. Federal Standard Fed. Std. 209E
Classification is based on the number of particles with a diameter of 0.5 μm or larger contained in one cubic foot (28.3L) of air.
JIS B9920 and ISO 14644-1
Classification is based on the number of particles with a diameter of 0.1 μm or larger contained in one cubic meter of air.
Safety Standards and Facility Hygiene Management at Our Culture Facility


The CPC (Cell Processing Center) is a cleanroom—a space where airborne microparticles and microorganisms are managed at an extremely high level of cleanliness. All materials, chemicals, water, and other supplies provided within that space are also managed to ensure no impurities are introduced.
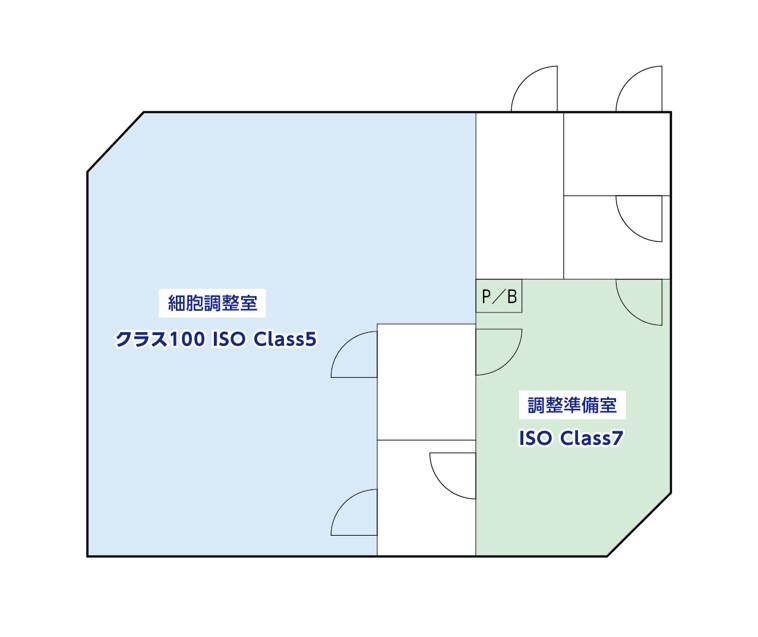
Since the introduction of dust from outside the room most often occurs via humans, the wearing of “clean suits” (resembling spacesuits) is mandatory when entering the cleanroom. Inside the CPC, there are two areas: the “Preparation Room” and the “Cell Processing Room.” Each area has its own designated cleanliness level. We manage operations through both hardware (structural equipment) and software (operational rules and education) to ensure processing and manufacturing can be carried out in a hygienic environment.

At Biostyle Clinic, we culture cells using “2D culture.” In 2D culture, stem cells grow spread out thinly without overlapping. This allows all cells to be uniformly exposed to the culture medium components, keeping stress from nutrient or oxygen deficiency to a minimum. This cultivation method is made possible by our dedicated culture specialists. All of these tasks are performed inside a safety cabinet, and the dispensing of the medium is also conducted within this cabinet.

Regarding laboratory techniques, accuracy and speed are vital, requiring proficiency through extensive experience. It is crucial to use cells from an optimal medium (cells that have divided fewer times), as cells have a division limit and gradually degrade (loss of differentiation potential, etc.). Since different types of cells require different compositions, finding the right medium composition is essential. We use an optimally composed proprietary medium. While stem cells can be cultured in inexpensive commercial media, achieving the required quality is difficult. We constantly perform fine adjustments while monitoring the state of the cells. Stem cells can degrade due to nutrient depletion or cell-to-cell contact, which can cause them to secrete inflammatory cytokines. To prevent degradation, one could provide cells with abundant media and space, but this would dilute the concentration of growth factors they secrete. Our unique know-how lies in the ability to fine-tune various conditions, such as timing, to culture the cells at the limit where degradation does not occur.
Stem Cells Showing Degradation
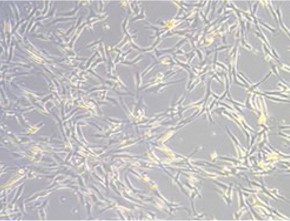
The cells appear flat with no thickness, showing signs of degradation. It is also likely that differentiation potential has been lost.
Healthy Stem Cell Growth
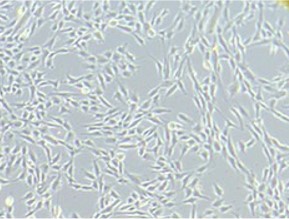
The cells are firm and tight, with a certain percentage of cells about to divide (white dots).
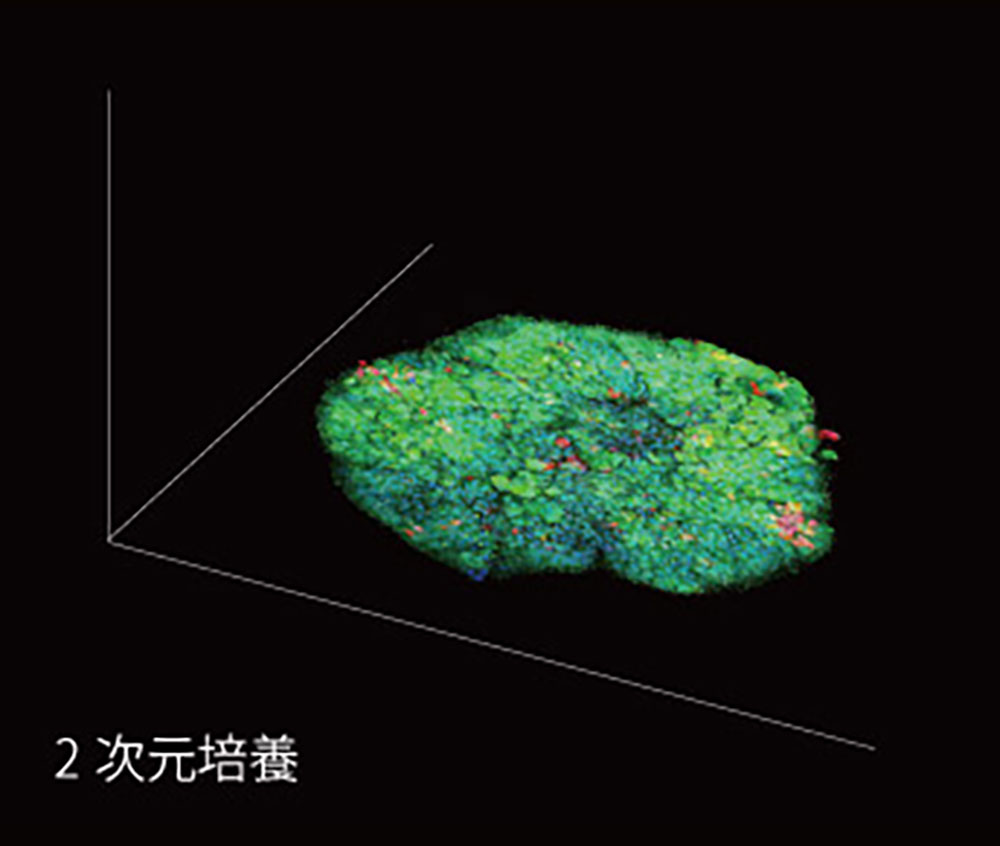
2D Culture
In 2D culture, cells are in planar contact with each other, and stimulus transmission between cells occurs only horizontally. The medium and reagents are supplied directly from above.
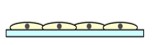
- Cell Shape:Flat and stretched out.
- Medium-Cell Contact:All cells are uniformly exposed to the medium components.
- Cell-Cell Junctions:Low frequency of junctions, differing from the physiological environment.
- Cell Differentiation:Cells do not differentiate much.
- Drug Sensitivity:High sensitivity; drugs show high efficacy.
- Cell Proliferation:Proliferation rate is higher compared to natural environments.
- Viability:High sensitivity to cytokines.
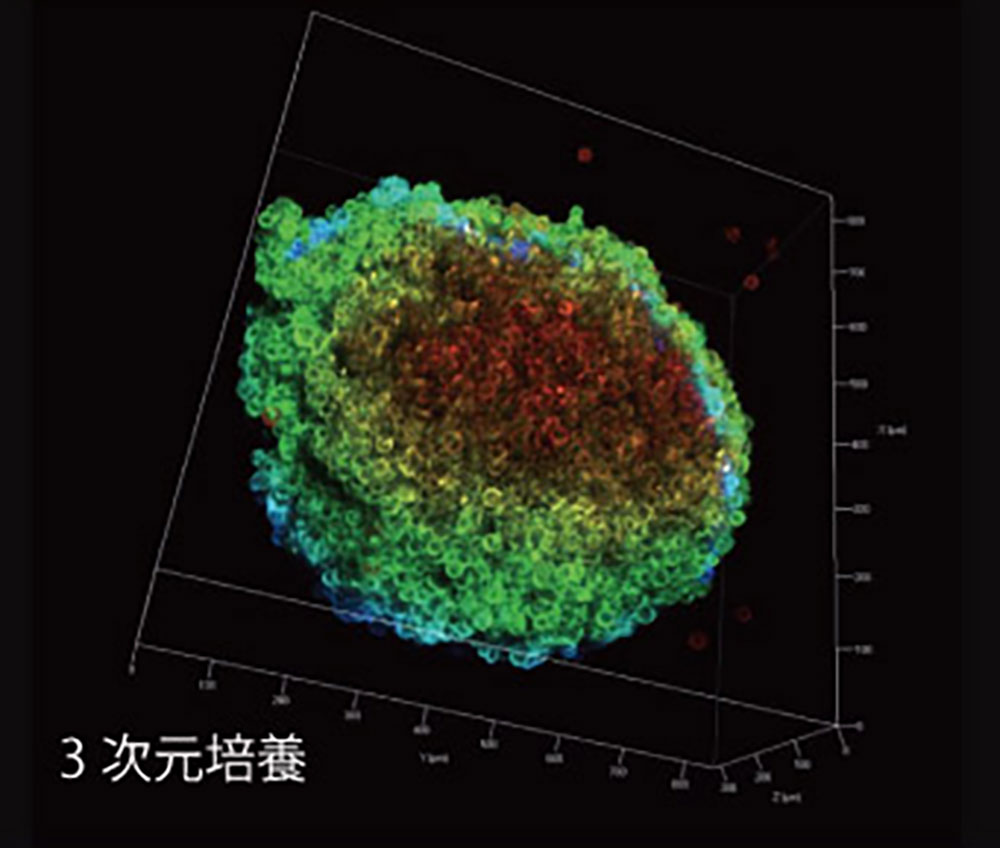
3D Culture
In 3D culture, cells are in three-dimensional contact, and stimulus transmission is stereoscopic. Internal cells are not in direct contact with the medium or drugs, which are supplied via diffusion.

- Cell Shape:Maintains a natural, oval shape.
- Medium-Cell Contact:Similar to physiological environments, there is a concentration gradient in the surrounding medium. Cells in the surface layer are exposed to more components than those in the deeper layers (non-uniform exposure).
- Cell-Cell Junctions:Widespread junctions allow for inter-cellular communication.
- Cell Differentiation:Steady differentiation of cells occurs.
- Drug Sensitivity:Cells often show resistance; drug efficacy is lower.
- Cell Proliferation:Rates fluctuate depending on cell type and 3D culture technology.
- Viability:High viability and low sensitivity to external factors.

Observation via microscope is conducted using a microscope within the cleanroom, where the status of cells being cultured in flasks is monitored.

The CO2 incubator in the cleanroom maintains a constant temperature and CO2 concentration by injecting carbon dioxide at a set temperature. This allows for cell culture, experiments, and observation under physiological conditions. The CO2 concentration is adjusted to 5%, close to the body’s partial pressure of CO2. This maintains the optimal pH (7.1–7.4) and temperature (37°C) for cell growth, while measures such as humidification and mold prevention are taken to keep the medium from drying out.

Supervising physicians and dedicated culture specialists monitor the cell status for each client individually. The reason we can perform 2D culture—which requires significant effort and thorough management—is that it allows us to fine-tune conditions to ensure cells are ready on the exact day of administration without degradation. On the day of administration, the supervising physician will explain the status of your cells based on a culture report while viewing cell images.

Since the cells we handle are ultimately used by end-users in medical settings, extremely high quality is required. Therefore, we culture under a strict quality control system to provide supernatants that are safe and effective. It is also important to accurately understand the state of the cells during culture and provide an optimal environment. To achieve this, we rigorously manage parameters such as the amount and duration of protective agents used for freezing and thawing, regular cell inspections, cell activity measurements, and the management of media and culture equipment. Furthermore, we believe it is important to treat cells with care while they are alive. By carefully performing the culture by hand and constantly checking the state and growth of the individual cells, we can cultivate high-quality cells. In the unlikely event that abnormalities are found during culture, the process will be halted as they cannot be used for treatment. In such a case, it will be necessary to collect tissue again; we ask for your understanding in order to ensure a safe and effective treatment.
Biostyle Clinic Chief Cultivator
Yoshichika Yamaguchi
After working at the Kishimoto Clinical Science Research Institute / Tokiwa Hospital (Immunology Section Cell Culture Room) and Japan Culture Laboratory Co., Ltd., he currently serves as the Head of Culture Technology at SCC Lab Co., Ltd. and as the Chief Cultivator at Biostyle Clinic.